DELIVERY PLATFORMS
SAMiRNA® (Self-Assembled-Micelle Inhibitory RNA)
SAMiRNA® is made of DNA/RNA heteroduplex siRNA core with conjugated hydrophilic four Hexaethylene glycol (HEG) and hydrophobic hydrocarbon tails at each end, which forms spontaneously a spherical and neutral nanoparticle in the aqueous solution.
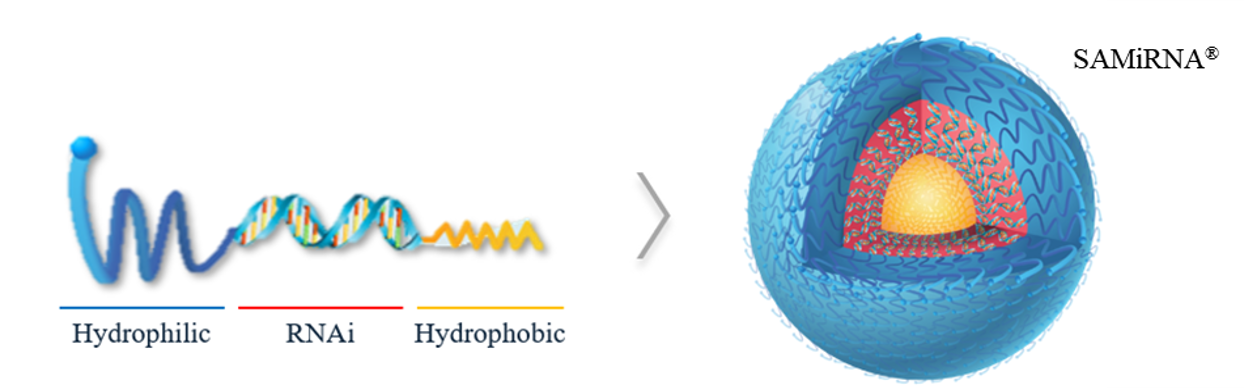
SAMiRNA represents a significant advancement in siRNA-based therapeutics, addressing many common challenges associated with this drug class.
siRNA drugs offer high target specificity and the ability to silence virtually any gene. They are easier to design than small molecules, allow flexible routes of administration, have low dosing frequency, and can provide sustained effects with manageable safety profiles.
However, conventional siRNAs are often unstable in vivo, prone to nuclease degradation, and can activate innate immune responses. Off-target effects and toxicity remain major barriers.
SAMiRNA addresses these limitations through optimized sequence design and a proprietary delivery platform.
The self-assembled micelle structure protects the siRNA from degradation while facilitating cellular uptake. Notably, off-target analysis confirmed that SAMiRNA does not induce off-target gene expression changes.
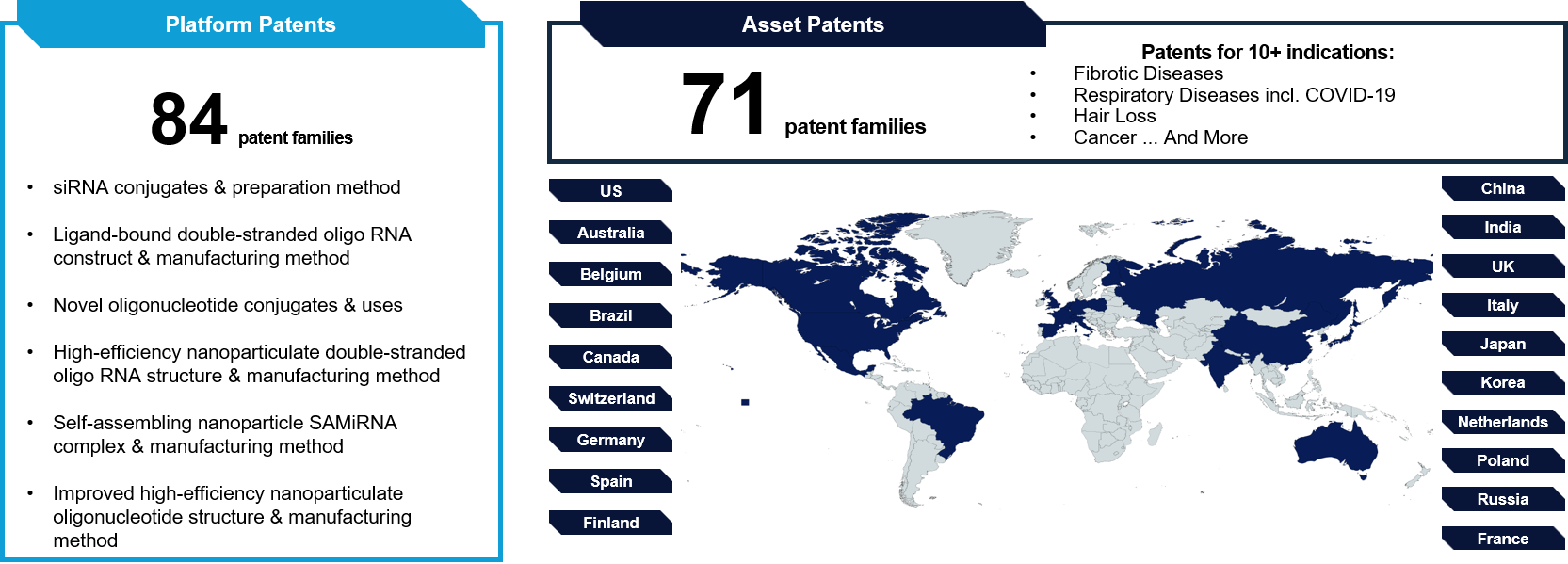
Intellectual Property
siRNAgen Therapeutics maintains a comprehensive intellectual property (IP) portfolio that reinforces our leadership and competitive advantage in the RNAi therapeutics market. Our granted and pending patents cover core aspects of the SAMiRNA technology, including amphiphilic molecular structures, siRNA conjugates, compositions, and therapeutic applications across multiple disease indications.
Our IP strategy includes:
Protecting the unique self-assembling micelle structures that form the foundation of the SAMiRNA platform for efficient delivery.
Securing the use of SAMiRNA in the treatment of specific diseases, including fibrosis and cancer.
Covering proprietary formulations for targeted siRNA delivery and novel composition.
This robust IP portfolio enables siRNAgen to pursue strategic partnerships, license our technology, and safeguard our innovations across key global markets. Ongoing global patent filings continue to expand the scope of SAMiRNA applications, further strengthening our position in the rapidly evolving RNAi therapeutics landscape and supporting our mission to address unmet medical needs.