1. Customized In vivo Study Design Based on Specialized Disease Models
siRNAgen Therapeutics offers validated rodent disease models for a wide range of indications, including inflammation, tumors, metabolic disorders, and kidney diseases. We provide optimized, customized study designs and execution tailored to specific research objectives.
2. Expertise in RNAi Therapeutic Evaluation for Gene Targets
Leveraging specialized expertise in RNAi-based therapeutic development, we precisely conduct siRNA/SAMiRNA-induced gene expression knockdown studies and downstream biomarker analyses to enable functional target validation and accurate efficacy assessment.
3. Quantitative Efficacy and Toxicity Assessment Through Histopathology
Following the administration of test substances, major organs are collected for histopathological analyses such as H&E and IHC staining. This enables both quantitative and qualitative evaluation of drug efficacy and toxicity at the tissue level.
4. Wide Coverage of Disease Models and Evaluation Parameters
A broad range of disease models—such as diabetes (db/db), obesity, NAFLD, cancer (xenograft), pulmonary fibrosis, and depression—can be utilized, along with diverse evaluation parameters including body weight, blood glucose, and blood/tissue biomarkers, enabling support for a wide spectrum of research needs.
5. Fast and Transparent Reporting System
From sample analysis to report delivery, we provide rapid and accurate result reports (including IND-supporting documentation) through a standardized operational system and experienced professionals. Customized reports can also be provided upon client request.
6. Efficacy Evaluation Center
The Efficacy Evaluation Center is located in Munpyeong-dong, Daejeon, and spans approximately 330 m2. It consists of four animal housing rooms, two necropsy rooms, and one pathology room. The facility can accommodate up to 1,500 rodents, all housed in individual cages to minimize cross-contamination and cross-infection, ensuring the highest research quality for our clients.
1. Animal housing (E.C.R.S)

2. Animal experiments (Biohazard Safety Cabinet)

3. Histopathological Analysis Equipment

4. Histopathology Equipment
(Microtome, Cryostat, Tissue Embedding Center, Tissue Processor, Auto-stainer)

5. Confocal microscopy system

Confocal microscopy image examples
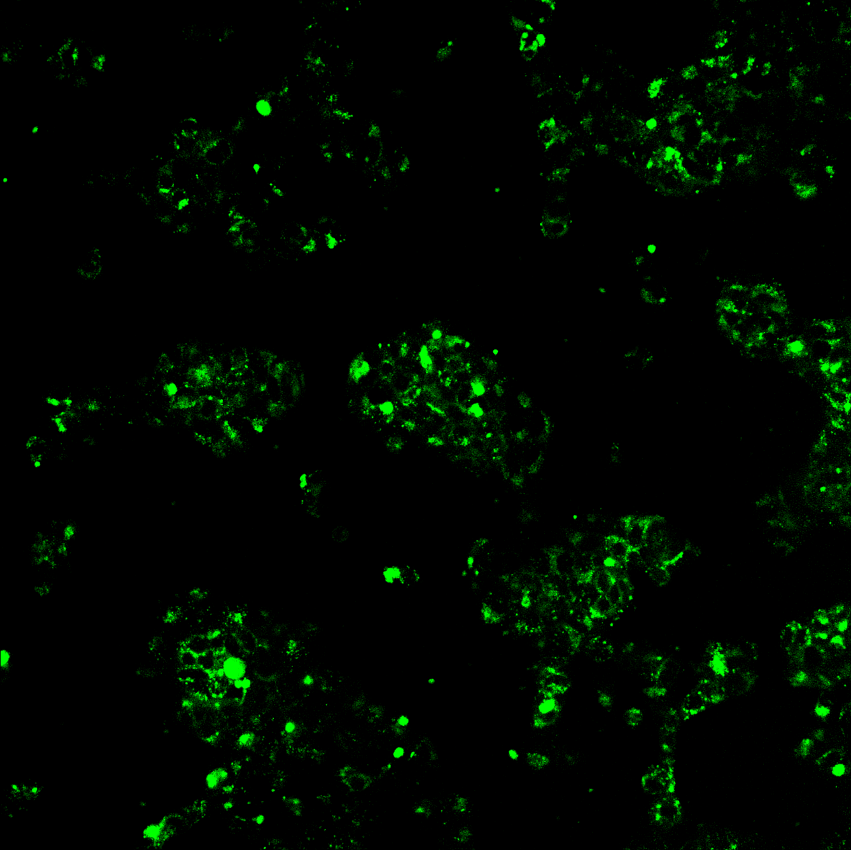
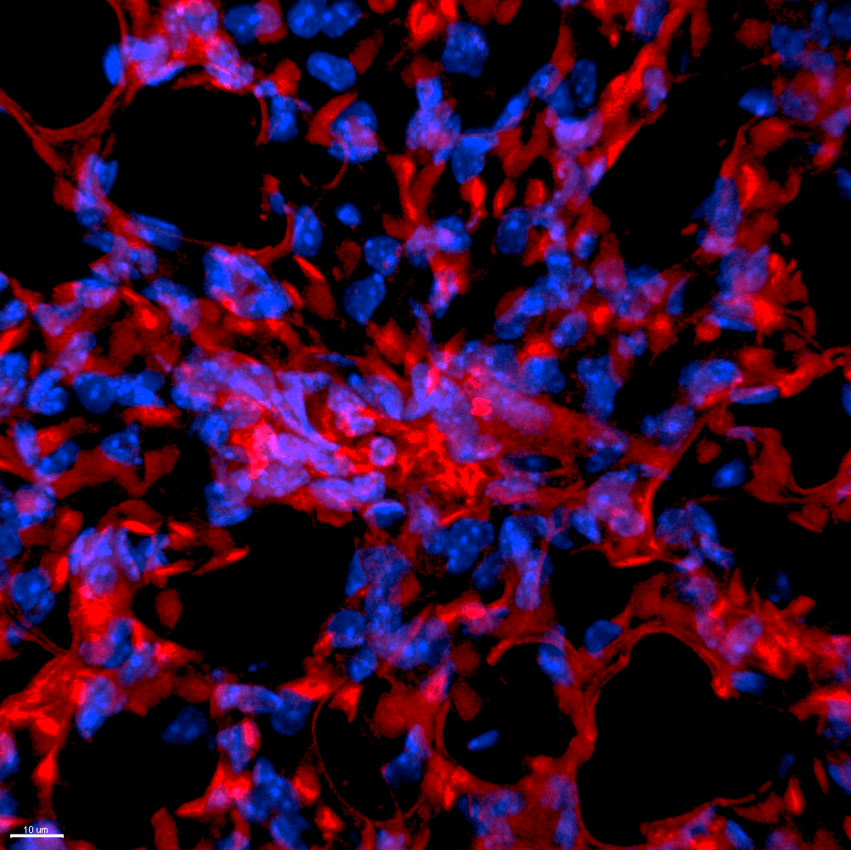
If you contact us (orders@sirnagen.com), we will promptly provide you with information on oligonucleotide synthesis specifications, scale options, and a quotation.