
Automated nucleic acid extraction system

Real-time quantitative thermal block for 384 or 96 well RT-qPCR
1. Reliable results with MIQE guidelines
The MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) guidelines establish the essential standards and information required for the accurate and reliable reporting of quantitative real-time PCR experiments.
All experimental procedures and data analyses in this RNAi High-throughput screening service are conducted in strict accordance with these guidelines.
By adhering to internationally recognized MIQE standards, the objectivity and reliability of the results are greatly enhanced, ensuring that the generated data are suitable for scientific publication.
2. High-throughput screening
High-throughput screening is conducted using the ExiPrep™ 96 Lite automated nucleic acid extraction system and the Exicycler™ 384 real-time quantitative thermal block for 384-well RT-qPCR.
The integration of these automated systems ensures high operational efficiency and supports large-scale sample processing.
3. End-to-End solution
By conducting synthesis as well as both in vitro and in vivo efficacy evaluations in one place, you can significantly reduce both time and costs.
4. Expertise in siRNA Drug Development
We possess extensive know-how in siRNA drug development, ensuring reliable and effective research outcomes.
Conventional algorithm-based designs may overlook certain highly potent drug candidates during the selection process.
Our service overcomes this limitation by sliding 1 nucleotide at a time across the target gene to generate all possible candidates, then thoroughly analyzing off-target effects and sequence similarity with model organisms to select as many candidates as possible.
This approach enables us to identify all highly effective candidates that may be overlooked by traditional methods.
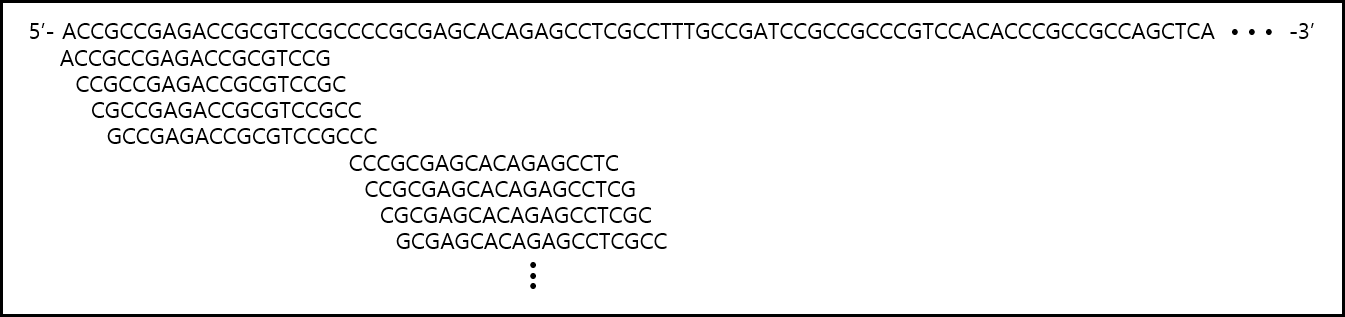 Figure1. An example of identifying target gene drug candidates using a 1-nt sliding window algorithm.
Figure1. An example of identifying target gene drug candidates using a 1-nt sliding window algorithm.
Testing is conducted in accordance with the MIQE guidelines and utilizes automated equipment, enabling the rapid acquisition of highly reliable data.
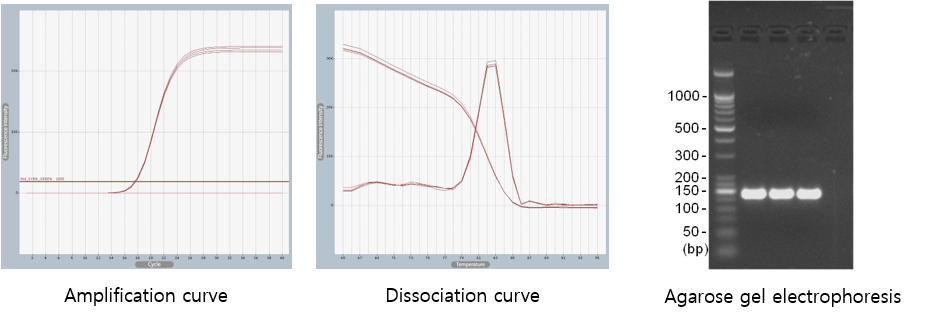 Figure2. Validation of selected primer sets. (Shown for human ACTB gene)
Figure2. Validation of selected primer sets. (Shown for human ACTB gene)
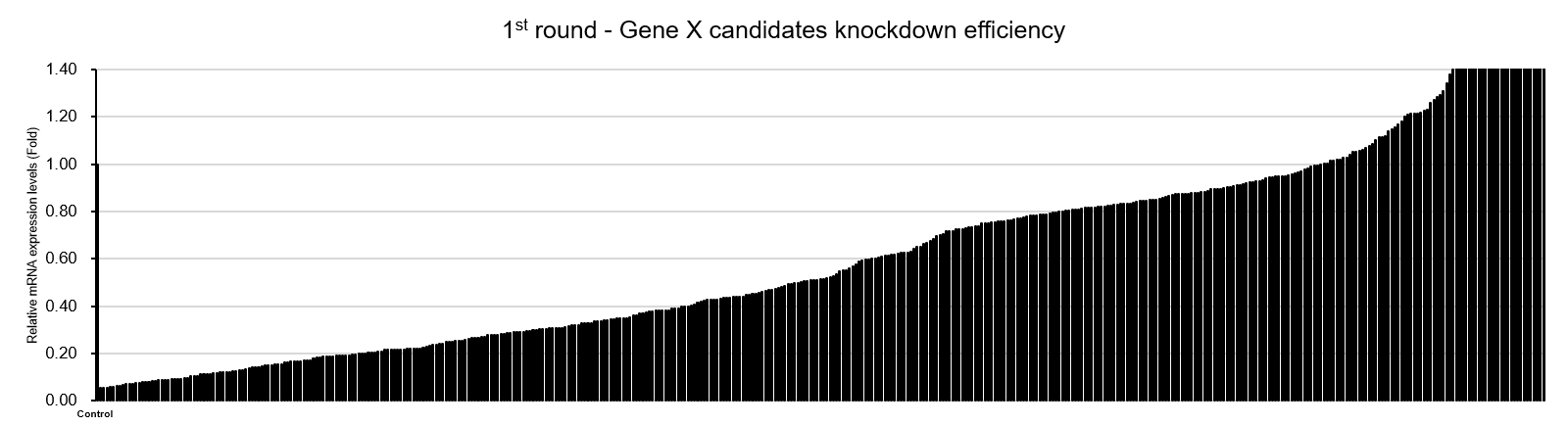 Figure3. Results of high-throughput screening of 448 SAMiRNA candidates targeting Gene X
Figure3. Results of high-throughput screening of 448 SAMiRNA candidates targeting Gene X
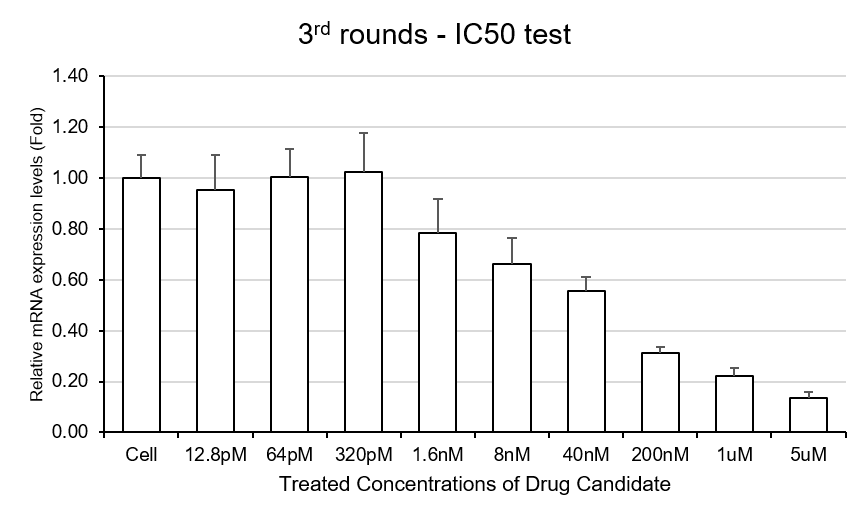 Figure4. Results of IC50 assay of SAMiRNA candidates targeting Gene X.
Figure4. Results of IC50 assay of SAMiRNA candidates targeting Gene X.
Our Pro-inflammatory Cytokine Response Test is a reliable method for verifying the induction of inflammatory immune responses and immunotoxicity.
The test analyzes changes in pro-inflammatory cytokine expression using RT-qPCR after treating human PBMCs with candidate compound.
This service allows you to scientifically demonstrate the safety of your candidates during the drug development stage.c.
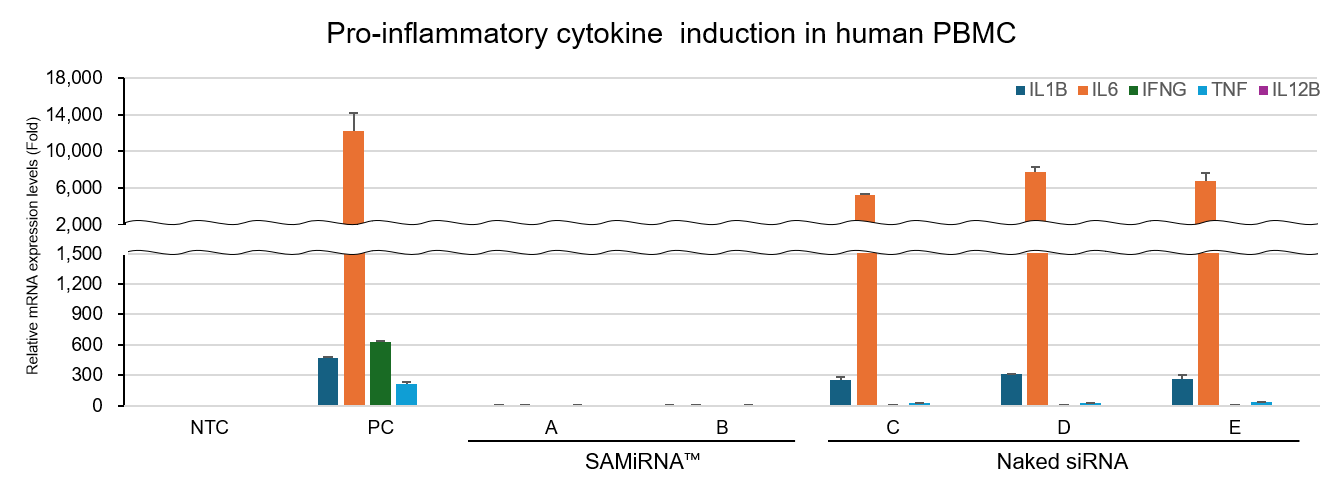
Figure5. Results of pro-inflammatory cytokine response test in human PBMCs using RT-qPCR
Our cytotoxicity tests provide an assessment of the safety of drug candidates using WST-1 and MTT assays.
Testing is performed using the standard MRC-5 cell line along with a diverse range of human-derived cell lines.
This approach allows us to provide toxicity results that are precisely tailored to your unique research goals.
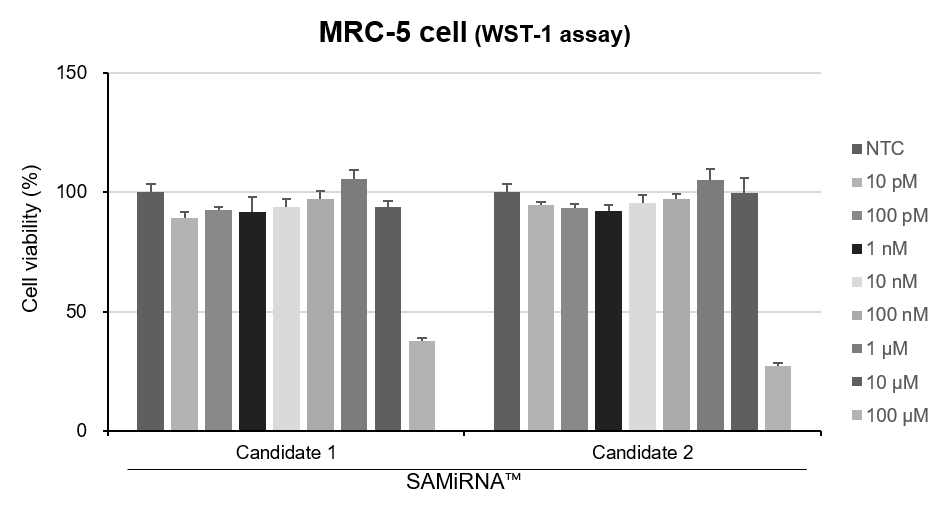
Figure6. Cytotoxicity test results with WST-1 assay in MRC-5 cells.
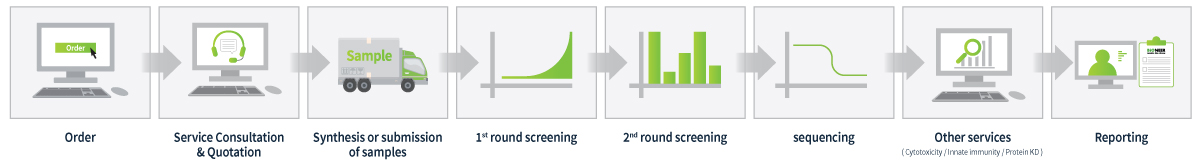
| Name | Content |
|---|---|
| Design & synthesis | Naked/Modified siRNA: On sequences between human and monkey by default. You can add animals such as mouse, rat, etc. |
| High-throughput screening (RT-qPCR) |
Screening experiment setup Obtain suitable primers and cell lines for the experiment. |
|
1st round screening: Screening all drug candidates |
|
|
2nd round screening: Screening the top candidates for selection |
|
|
3rd round screening: Top 5 candidates for IC50 testing |
|
| Other |
|
* All assays are performed in triplicate for both biological and technical replicates.
If you contact us(orders@sirnagen.com), we will promptly provide you with information on the experiment design and a quotation.
If you are interested in utilizing our SAMiRNA™ platform, please contact us for further information.